#19 Metabolic Acidosis w/ NephMadness
In this Critical Care Time x Neph Madness collab, Nick & Cyrus host Drs. Tim Yau and Jeff Kott for a comprehensive discussion of metabolic acidosis with a focus on critically ill patients. We start by outlining a pragmatic approach to acid/base derangements peppered with some fact finding and myth busting, CCT style! We then turn our attention to working up and treating metabolic acidosis in the ICU. If you are looking for a one stop shop when it comes to metabolic acidosis in the ICU - look no further. You may even figure out what Spanx & sodium bicarb have in common!
Follow Dr. Jeff Kott via X (@jrkott27) and Dr. Tim Y au via A (@Maximal_change). Check out the AJKD blog, NephMadness 2024 page here!
Shownotes by Dr. Shane McMahon:
Quick Take Home Points:
Metabolic acidosis is the most common acid-base derangement in the intensive care unit.
Mind the pH - that’s the first step in determining the primary disorder.
Next, address compensation - or lack there of - using the formulas for compensation. Check out our infographic for more details!
Finally, use delta-delta so you don’t miss an additional disorder.
Once the acid-base status is determined and differentials are considered. the primary goal for the clinician should be correcting the cause of the acid-base derangement,
Sodium bicarbonate boluses & infusions are helpful to temporize but rarely will treat the problem and it’s not without risks - such as exacerbating tissue hypoxia.
Renal replacement therapy can be used as a temporizing measure in certain cases - especially in cases of oliguric or anuric renal failure.
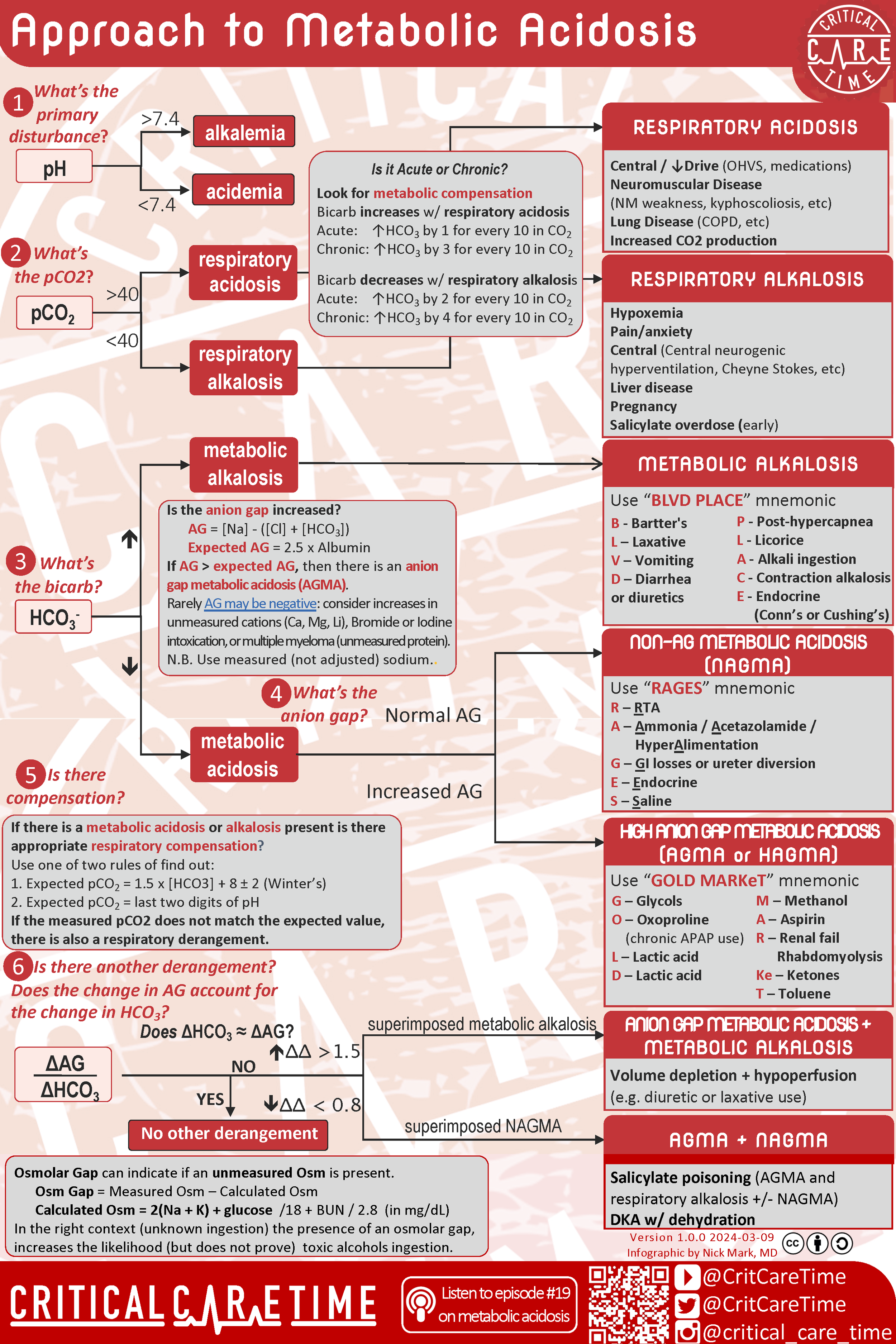
Infographic:
Infographic summarizing a systematic approach to Metabolic Acidosis.
Show Notes:
Approach to Acid/Base & Metabolic Acidosis
First Step in assessing Metabolic Acidosis: Look at the pH!
Can be from ABG or VBG
Definition of normal pH : 7.35 - 7.45
IF there is an underlying alkalosis or acidosis then the pH will be as follows:
Acidosis < 7.4
Alkalosis >7.4
Beware of automatically associating certain bicarbonate levels with acidosis or alkalosis
For example, a low bicarbonate can indicate metabolic acidosis or may also represent appropriate compensation for a respiratory alkalosis
Although not perfect, a thorough History and Physical Exam helps to build an accurate differential for detected acidosis/alkalosis. For example, diabetic patients can be associated with ketoacidosis, psychiatric patients may have ingested substances with toxic/acidic metabolites, a Review of Systems positive for recurrent diarrhea may indicate GI bicarbonate losses
Medication review is extremely important! For example:
SGLT-2 Inhibitors i.e. Empagliflozin, Dapagliflozin are associated with Euglycemic Diabetic Ketoacidosis (DKA)
Ingestion of many Bicarbonate containing antacids leads to Milk-Alkali Syndrome
Patients taking Carbonic Anhydrase inhibitors (Acetazolamide) will have renal loss of Bicarbonate with associated metabolic acidosis
Physical Exam findings associated with acid-base derangements include Asterixis due to Uremia from renal dysfunction and Optic Neuropathy (blindness) which can be secondary to Toxic Alcohol Ingestion.
Which labs should we be ordering to evaluate for acid-base derangements?
Classically the first two tests to order are a blood gas (ABG or VBG) and a serum chemistry panel. The blood gas reveals the overall pH and partial pressure of CO2 (pCO2) of a patient's whole blood while the serum chemistry gives you an actual serum bicarbonate (as opposed to a approximated bicarbonate on a whole blood blood gas). In general, these two tests can be interpreted in the following ways: Remember Low pH < 7.4 & High pH >7.4. Low PpO2 < 40 mmHg & High PCO2 > 40 mmHg
Low pH & Low Bicarbonate - Metabolic Acidosis
Low pH & High pCO2 - Respiratory Acidosis
High pH & Low pCO2 - Respiratory Alkalosis
High pH & High Bicarbonate - Metabolic Alkalosis
After categorizing a patient’s acid-base disturbance further evaluation is possible and can help build a differential for the etiology of the acid-base disturbance.
When a patient’s primary disturbance is a metabolic acidosis, calculation of the patient’s Anion Gap allows sorting into major buckets of Anion Gap Metabolic Acidosis (AGMA) or Non-Anion Gap Metabolic Acidosis (NAGMA). The variables for this equation can be found from either peripheral blood gasses or serum chemistry studies. Typically a serum bicarbonate (HCO3) is directly obtained from a serum chemistry test due to increased accuracy as opposed to the calculated HCO3 from a blood gas. That being said, these differences are overall minor and blood gasses can be utilized to calculate the anion gap if future measurements are also derived via blood gasses. Consider Serum Chemistry assays apples and whole blood blood gasses oranges! Make sure to compare apples to apples and not mix in those oranges! Luckily the calculation of anion gap is very straightforward as seen below:
Anion Gap = [Na] - {[Cl]+[HCO3]}
Normal Anion Gap = 8-12 or 2.5 x the serum albumin (this is particularly important in low albumin states)
As (simplified) rule:
Anion Gap > 12 w/ metabolic acidosis —> AGMA
Anion Gap <12 w/ metabolic acidois —> NAGMA
Breaking into AGMA vs NAGMA allows you to formulate a differential diagnosis:
AGMA: MUDPILES (old school mnemonic)
M - Methanol
U - Uremia
D - DKA
P - Propylene Glycol/paraldehyde
I - Iron / Isoniazid
L - Lactic acidosis
E - Ethylene Glycol
S - Salicylates
AGMA: GOLDMARKeT (new school mnemonic)
G – Glycols
O – Oxoproline (chronic APAP use)
L – Lactic acid
D – Lactic acid
M – Methanol
A – Aspirin
R – Renal failure / Rhabdomyolysis
Ke – Ketones
T – Toluene
NAGMA: RAGES mnemonic
R – RTA
A – Ammonia / Acetazolamide / HyperAlimentation
G – GI losses or ureter diversion
E – Endocrine
S – Saline
Once an acid-base disturbance has been identified and characterized it is next important to evaluate for how chemically compensated the patient is. To review basic acid-base biochemistry recall that:
Serum Acid [H+ ions] + Serum [HCO3] ⇄ Dissolved [CO2] + Serum [H2O]
As one substrate increases i.e. acid/H+ there is a chemical momentum to shift to conversion of the H+ into CO2 and H2O. This teeter-totter effects both sides of the chemical equation allowing for a homeostasis around pH 7.4.
With understanding the primary mechanism of acid-base compensation we are now able to calculate an expected PCO2 with a given [HCO3]. For anion gap metabolic acidosis this is done via Winter’s Formula:
Expected PCO2 range = 1.5 [HCO3] +8 ± 2
If actual PCO2 (measured on blood gas) is greater than expected PCO2 then there is extra CO2 in the blood independent from the metabolic acidosis. This indicates a concomitant respiratory acidosis.
If actual PCO2 (measured on blood gas) is within than expected PCO2 range then the body is appropriately compensating.
If actual PCO2 (measured on blood gas) is less than expected PCO2 then there is a concomitant respiratory alkalosis.
Evaluating for appropriate compensation from a primary respiratory disturbance is more brute memorization however the 1-2-4-5 rule allows for quick interpretation of many respiratory disorders. The 1-2-4-5 rule takes advantage of the fact that for every change in pCO2 of 10 mmHg there should be a corresponding change in [HCO3]. The amount of expected change in serum [HCO3] depends on if this is a acute (<2 days) or chronic (> 2 days) process: (i.e. has the kidney had the opportunity ot compensate)
| [HCO3] (Baseline = 24) | ||
|---|---|---|
| Every 10 mmHg change in pCO2 (Baseline = 40 mmHg) | ACUTE | CHRONIC |
| ↑PaCO2 (Resp. Acid.) | ↑ 1 | ↑ 4 |
| ↓PaCO2 (Resp. Alka.) | ↓ 2 | ↓ 5 |
Example: For an acute respiratory acidosis with pCO2 of 60 there is a 20 mmHg increase in pCO2 from baseline. That corresponds with an expected serum HCO3 increase of 2 above baseline to 26.
Finally, after categorizing the acid-base disturbance and evaluating for compensation it is sometimes required to evaluate for a third acid-base disturbance. This is needed when the above calculations indicate undercompensation or overcompensation which may indicate an additional acid/base process.
This can be calculated via the “Delta-Delta” which is (Expected anion gap - Actual anion gap)/(Expected [HCO3] - Actual [HCO3])
A simple pneumonic for this is “A over B” or Anion gap over Bicarbonate
Quick Interpretation of the “Delta-Delta” or “Delta Gap” is as below:
“Delta-Delta” between 0.4 - 1.0 = Mixed AGMA + NAGMA
“Delta-Delta” between 1.0 - 2.0 = Pure AGMA
“Delta-Delta” > 2.0 = AGMA + Metabolic Alkalosis
The basis for this equation is that we should be able to calculate a expected serum bicarbonate for a given AGMA with known pCO2. If the actual serum bicarbonate is lower than expected that indicates a co-existing NAGMA.
Another approach is the “bicarb before” - Take the patient’s anion gap (in a HAGMA) and subtract the normal gap (estimated to be 10 in most folks)
Take that value and add it to the patient’s bicarbonate
This “corrects” for the AGMA - if the result is within 2 of normal (22-26), there is no other disorder
If the “bicarb before” calculation is <22, there is likely a NAGMA present as well
If the “bicarb before” calculator is >26, there is likely metabolic alkalosis present as well
Summary of the above approach:
Remember that pH is -log10 of [H+] in the blood. 7.4 is generally equal to a [H+] of 40 nMol/Liter.
Remember:
If dealing with a respiratory issue the kidneys should be trying to correct
If dealing with a metabolic issue the lungs should be trying to correct
Anion Gap, Osmolar Gap, & Delta-Delta
What exactly is an Anion Gap? What is the difference between an AGMA and a NAGMA?
Anion Gap is based on the idea that the body’s positive (cations) equal the body’s negative (anions).
Hydrogen is the body’s main cation
Chloride and Bicarbonate are the bodies main anions
Generally, a normal gap is between 10-12 however this can change!
For example, for every 1 g/dL a serum albumin is lowered, there is a corresponding decrease in expected anion gap by 2.5
If patient has serum albumin is 3 then expected AG is 7.5-9.5 as opposed to 10-12
Other causes of altered expected AG are: Hypercalcemia, hypermagnesemia, bromide containing medications (interferes with calcium measurement)
In an Anion Gap Metabolic Acidosis there are one or more additional anions (negatively charged ions) contributing to the excess [H+] in the body.
In a Non-Anion Gap Metabolic Acidosis there is a loss of [HCO3], which leads to a increased [H+] as the acid-base equation shows. There is a push to replenish HCO3 which leads to increased H+ generation with a skewed and increased [H+]:[HCO3] ratio. More [H+] than [HCO3] leads to acidosis/acidemia.
For example: in diarrhea you have loss of bicarbonate in stool, in Renal Tubular Acidosis (RTA) there are urine bicarbonate losses
In the ICU setting most acidoses we see are AGMAs.
What is a “Negative Anion Gap”?
This refers to elevated blood levels of positively charged paraproteins such as seen in Multiple Myeloma and other gammopathies. Increase in unmeasured positive proteins leads to a compensatory increase in chloride, resulting in a negative anion gap.
Keep this in mind in patients with significant AGMAs and concomitant gammopathies as their true Anion Gap may be much higher than it appears.
What about Hyperglycemia and measurements of Anion Gap?
When calculating Anion Gap in patients with hyperglycemia use the actual measured sodium concentration, not the corrected concentration!
Sodium is adjusted for hyperglycemia to accommodate for osmotic shifts due to changes in osmotic pressure from sugar molecules. Remember that Glucose is electrically neutral and thus does not affect anion gap!
Corrected Sodium = [Na] + (1.6)(N) ; N = integer of number of times there is an extra 100 serum glucose over baseline.
For Example, if [Na] = 130 and Glucose is 600 mg/dL then corrected sodium is 130 + (1.6 x 5) = 138 mMol. 600 mg is 5 (100mg/dL) higher than 100 mg/dL baseline.
Looking at Lactate/Lactic acid. If a patient is generating lactic acid due to ischemia, then the H+ ion that the molecule of lactic acid produces is buffered by a molecule of HCO3-. This leaves a negatively charged lactate molecule (Anion) which then accumulates and can be indirectly measured as part of the anion gap!
Any disorder where there is an unmeasured anion (ketoacids in DKA, toxic alcohols, lactic acid, uric acids) will lead to buffering by HCO3- with resulting Anion Gap.
Mythbusting Lactate vs Lactated Ringers
Lactate is the conjugate base of lactic acid.
LR does not have lactic acid in it, rather it has lactate!
Lactic acid by itself is not of physiologic consequence as long as body can clear it!
This is performed by the liver and occurs on a daily basis as your body switches to majority anaerobic respiration.
Mind the (other) gap: Osmolar Gap and Urine Anion Gap
Osmolar Gap: Difference between directly measured and calculated expected serum osmolality.
Calculating expected serum osmolality.
Serum Osmolality = 2x[Na] + [BUN]/ 2.8 + [Glucose]/18
Normal serum osmolality is approximately 290 mOsm/L
If the difference (gap) between measured and expected(calculated) osmolality is 10 or higher, then the patient has a positive Osmolar Gap. This indicates other osmotically active substances in the blood like Ethanol, Propylene Glycol, Methanol, Ethylene Glycol, Isopropyl alcohol.
Most of these toxic alcohols cause a AGMA as well with the exception of Isopropyl Alcohol.
Remember: Isopropyl Alcohol causes osmolar gap without anion gap
Urine Anion Gap: Used to estimate whether kidneys are appropriately dumping out protons in the setting of acidemia. Kidneys do this by chemically bonding excess H+ ions to NH3 making ammonium, NH4+ for urinary excretion.
Urine Anion Gap = Urine Sodium + Urine Potassium - Urine Chloride
The kidneys typically match up cations with anions just like the serum so think of urine positives (Urine Sodium + Urine Potassium + Urine Ammonium) = urine negatives (Urine Chloride + Urine Bicarbonate)
Urine ammonium and bicarbonate are not directly measured so we need to infer their concentration by looking at the balance between the ions we do measure
Example: If urine Anion Gap is very positive then we know there are a lot of unmeasured anions in the urine AND/OR the amount of unmeasured ammonium must be very low
Increased urinary loss of Bicarbonate or failure to urinate ammonium are both indicative of RTAs!
Example: If urine Anion Gap is very negative then we know there are a lot of unmeasured cations (ammonium) and/or decreased urinary bicarbonate. This indicates proper kidney function when compensating for a systemic acidosis.
These calculations are estimations and may not be consistent between institutions and have limited clinical reliability.
Application and Interpretation of Acid-Base Derangements in Critically Ill while in the ICU
Seeing severe Acid-Base derangements is common in the ICU and the treatment plan revolves about the underlying cause and accuracy of your interpretation of their Acid-Base dysfunction. Renal Replacement Therapy (RRT) is not the answer for a portion of severe Acid-Base derangements.
For example: Diarrhea leads to a pure HCO3 deficit which can be treated simply with HCO3 replenishment despite a possibly intimidatingly low blood pH on presentation.
Likewise, a severe AGMA in a patient with DKA requires insulin to truly treat underlying cause. Otherwise efforts to correct the pH with additional HCO3 or RRT will be temporarily beneficial, if at all.
Lactic Acidosis is particularly common in the ICU setting representing how critically ill these patients are. Always keep a close eye on lactic acid levels as a general marker of perfusion and hepatic function.
If a patient has cancer be aware of the Warburg Effect which describes the ability of these cancer cells to switch to anaerobic respiration en masse producing a significant lactic acidosis without perfusion deficits.
Other commonly found etiologies of metabolic acidosis in the ICU include ischemic gut/limb, poor perfusion of all distal tissues, DKA and severe renal failure.
With regards to bicarbonate supplementation and a severe low pH (defined as less than 7.2) there are small studies showing no significant increase in hemodynamics or decrease in mortality with administration of a bicarbonate drip.
The BICAR-ICU study out of France showed that patients with severe (<7.2) lactic acidosis had no difference in mortality with bicarbonate drip administration group versus no bicarbonate given; however, there was a signal for decreased mortality and need for RRT in patients with AKI given bicarbonate.
So, in the critical care setting the use of bicarbonate to temporize a unstable patient is reasonable however 1) this is a temporary measure like a bandaid and 2) No proven mortality benefit in use of bicarbonate in severely acidotic patients
Remember that bicarbonate is administered as an isotonic drip or via pushes of hypertonic saline rich volume. This can be detrimental to patients who cannot tolerate further fluid overload well i.e. heart failure patients. Additionally, watch out for electrolyte defects like hypocalcemia, hypokalemia with use of bicarbonate.
Try to correct any underlying hypokalemia or hypocalcemia prior to administration of bicarbonate
Also remember that, due to acid-base balance equation above, HCO3 will be converted into CO2. In patients with respiratory failure this can lead to CO2 retention and worsening respiratory acidosis.
RRT is not indicated for these patients as this is an underlying respiratory issue, not a bicarbonate deficiency
Finally, remember that O2 dissociates from hemoglobin better in acidic environments!
Imagine a hypoxic patient with a decreased SpO2. This SpO2 is decreased due to appropriate O2 offloading to tissues partly in response to their acidic tissues. If you now give amps of Bicarbonate and raise the peripheral pH then the SpO2 will increase due to less O2 offloading to the tissues. This actually potentially leads to worsening tissue hypoxia!
Is there an alternative to Bicarb? There USED to be…
Tris(hydroxymethyl)aminomethane is a previously available alternative to bicarbonate which can raise pH without the CO2 issues of bicarbonate. A downside to this therapy is that it is excreted via the kidneys, which are often dysfunction in the critical care setting.
Starting Renal Replacement Therapy
Typically Renal Replacement Therapy (RRT) is initiated if patients have refractory acidemia due to metabolic acidosis. It is helpful to think of dialysis as a big bicarbonate drip with the benefits of removing toxic metabolites like toxic alcohols.
RRT actually leads to increased lactate production and will not correct underlying lactic acidoses!
If you see a correcting lactate in while on RRT this actually indicates improving metabolic acidosis not just lactate clearance by dialysis.
There are different methods of RRT. Commonly we see Intermittent Hemodialysis (IHD) and Continuous Renal Replacement Therapy (CRRT).
IHD corrects acid–base disturbances faster than CRRT however has more impact on hemodynamics and can cause intradialysis hypotension more severely than CRRT. This depends on the patient’s underlying hemodynamic/cardiovascular reserve prior to initiating the dialysis circuit.
IHD corrects acidemia quickly due to much higher bicarbonate concentrations in the dialysate.
There are no trials indicating at what pH to start RRT!
Audio
Video
-
Jaber S, Paugam C, Futier E, et al. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (Bicar-icu): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet. 2018;392(10141):31-40.
Chow E, Clement S, Garg R. Euglycemic diabetic ketoacidosis in the era of SGLT-2 inhibitors. BMJ Open Diabetes Research and Care. 2023;11(5):e003666.
Nickson C, Nickson C. VBG versus ABG. Life in the Fast Lane • LITFL.
Uribarri J, Oh MS. The urine anion gap: common misconceptions. J Am Soc Nephrol. 2021;32(5):1025-1028.
Jeffrey Kraut, Nicoloas Madias. Lactic Acidosis: Current Treatments and Future Directions. Am J Kidney Dis. 2016. 68 (3) 473-482
Najem O, Shah MM, De Jesus O. Serum osmolality. In: StatPearls. StatPearls Publishing; 2024.
Nadler ST, Suri J, Swenson ER. How basic can you be? Annals ATS. 2019;16(8):1057-1061.
van Hoeven KH, Joseph RE, Gaughan WJ, et al. The anion gap and routine serum protein measurements in monoclonal gammopathies. Clin J Am Soc Nephrol. 2011;6(12):2814-2821.
Hopkins E, Sanvictores T, Sharma S. Physiology, acid base balance. In: StatPearls. StatPearls Publishing; 2024.
Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211-218.
-
ELAIN
AKIKI
AKIKI-2
IDEAL-ICU
STARRT-AKI
-