#57 Venous Decongesation, Volume Tolerance, and Bedside Assesssment
What is the microcirculation? How are terms like volume-status and fluid-responsiveness misleading? Join us as we sit down with Dr. Ross Prager to unpack and de-mystify the concepts of congestion and volume tolerance along with our survey of the tools of the trade as it relates to these terms. On this Critical Care Time episode we really get into the weeds on this stuff and even get theoretical at some points so it's NOT for the faint of heart! However, if you listen to us for the deep dives into physiology and if you want to level-up your ICU patient care, this is the episode for you! Listen, leave us some feedback and drop us a review!
Guest: Ross Prager, MD
Intensivist at London Health Sciences Centre | Assistant Professor at Western University
@ross_prager
3 Step Approach to Answer “Should I give IV Fluids”?
Are they in shock? (micro-circulation)
Are they fluid tolerant? (congestion assessment)
Are they fluid responsive? (likelihood of improving macro-circulation)
TL;DR (Key Takeaways)
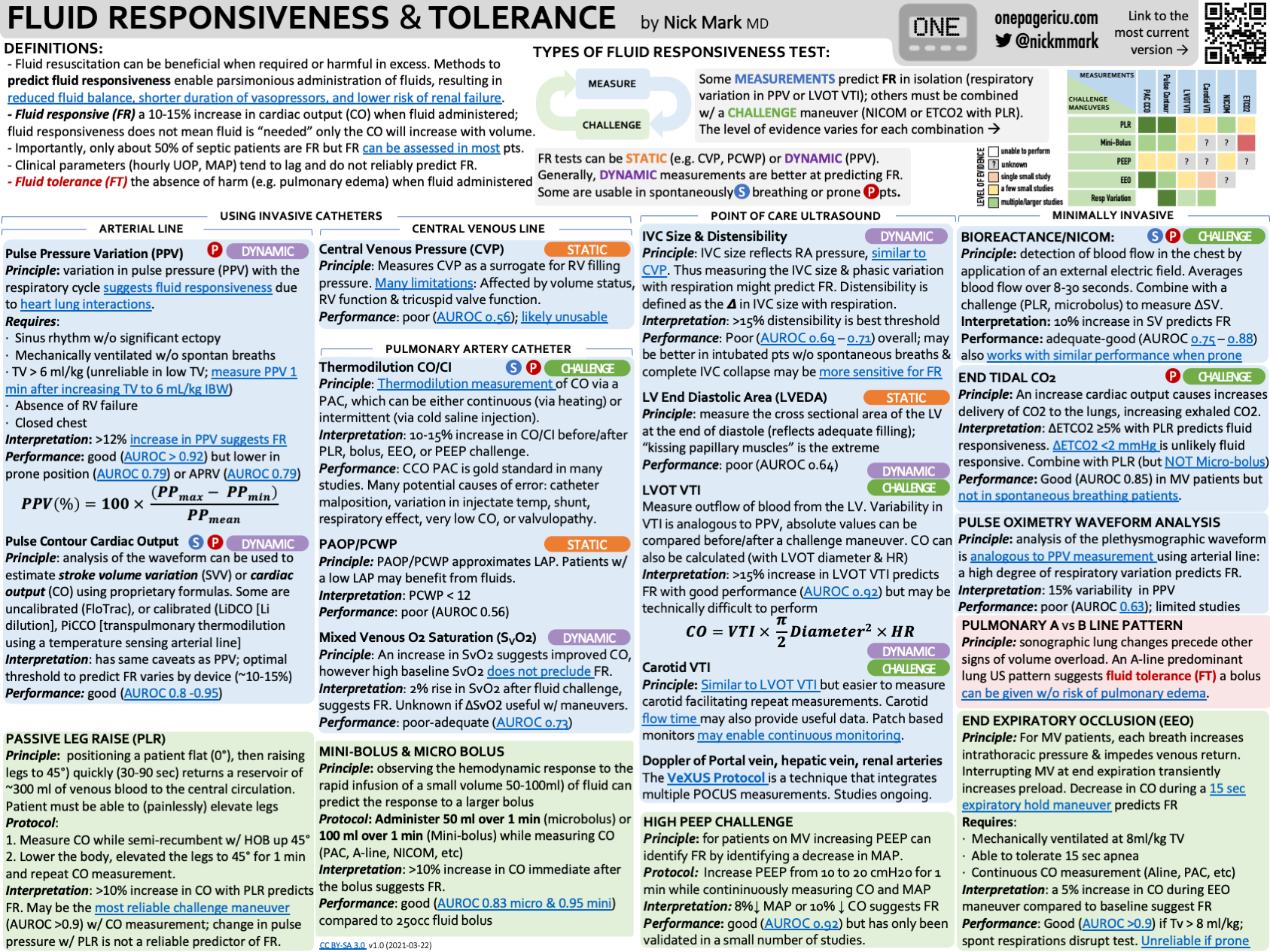
“Volume status” isn’t a single, standardized thing—so stop treating it like one. Instead, measure the physiology that maps to decisions: stroke volume (SV)/cardiac output (CO), fluid responsiveness, fluid tolerance, venous congestion, and (sometimes) mean systemic filling pressure (MSFP).
Microcirculation matters. Bedside capillary refill time (CRT) is a practical perfusion marker and, in sepsis, CRT-targeted resuscitation performed at least as well as lactate-guided care and may reduce organ dysfunction.
“Just give 500 mL and see” is not benign—positive fluid balance is consistently associated with worse outcomes (AKI, mortality). Use dynamic tests (PLR, PPV/SVV under the right conditions) and be deliberate.
POCUS is the workhorse: lungs (B-lines), focused echo (VTI changes with maneuvers), IVC/IJ (with caution), and VExUS (an integrated Doppler view of systemic venous congestion) to forecast AKI risk and guide de-resuscitation.
Arterial waveform dynamics (PPV/SVV) predict fluid responsiveness only if conditions are met (controlled ventilation, regular rhythm, adequate VT). Static measures like CVP don’t predict responsiveness.
PAC is now selective: valuable in complex shock (e.g., RV failure/pulmonary hypertension), but used less frequently.
Definitions (fast glossary)
Cardiac Output (CO): blood volume pumped by the heart per minute; CO = SV × HR
Stroke Volume (SV): blood ejected per beat
Fluid Responsiveness: ≥10–15% increase in SV/CO after a reversible preload challenge (e.g., passive leg raise or tiny “mini-bolus”), indicating the heart is on the ascending limb of the Frank-Starling curve
Fluid Tolerance: likelihood that a patient can receive fluid without harm (i.e., without worsening pulmonary/systemic congestion).
Venous Congestion: pathologic elevation of venous pressures causing organ edema & dysfunction (e.g., kidneys, liver, gut).
Right-sided congestion → systemic organs
Left-sided congestion → lungs.
Microcirculation vs Macrocirculation: macrocirculation = measurable big-vessel/heart variables (MAP, CO); microcirculation = tissue-level flow and oxygenation. Shock = inadequate tissue perfusion—hence CRT/lactate/mottling interest.
MSFP (Mean Systemic Filling Pressure): the upstream pressure for venous return; reflects the “stressed” volume (effective circulating volume that drives venous return). Vasopressors can recruit unstressed → stressed volume by decreasing venous capacitance.
Start at the Microcirculation: Capillary Refill Time (CRT)
ANDROMEDA-SHOCK (JAMA 2019): Early septic shock randomized to CRT-targeted vs lactate-targeted resuscitation. Similar or better outcomes (trend to lower mortality; faster organ-dysfunction resolution in Bayesian reanalysis) and fewer “overtreatment” signals in the CRT arm. Practical, free, repeatable.
Subsequent analyses suggest stopping fluids once CRT ≤3 s is safe, with comparable microcirculatory surrogates.
What is Mean Systemic Filling Pressure?
Mean Systemic Filling Pressure (MSFP) is the pressure in the entire systemic circulation when the heart stops and flow falls to zero—i.e., the pressure you’d measure after arterial and venous pressures equilibrate. It reflects how much of the circulating volume is “stressed” (the portion that actually stretches the vasculature and creates pressure) given the venous capacitance/compliance.
Why it matters: MSFP is the upstream driving pressure for venous return.
Venous return (VR) follows: VR = (MSFP − RAP) / R_vr,
where RAP is right atrial pressure and R_vr is resistance to venous return. At steady state, CO = VR.
What raises MSFP:
Fluids: add to stressed volume → ↑ MSFP.
Venoconstrictors (e.g., norepinephrine): recruit “unstressed” → stressed volume by shrinking venous capacitance → ↑ MSFP (often called “autotransfusion”).
Venodilators: do the opposite (↓ MSFP).
Clinical pearls:
You can’t directly measure MSFP at the bedside (it’s a zero-flow concept). Surrogates/estimators (e.g., P_msa models using MAP/CVP/CO) exist but aren’t universally adopted.
CO rises when you increase the gradient (MSFP − RAP). If a fluid bolus raises RAP as much as MSFP, the gradient (and thus CO) may not improve—explaining why some “test boluses” fail.
Vasopressors can improve CO in vasoplegic states by increasing MSFP (recruiting stressed volume) even without giving fluid—useful in patients with low fluid tolerance.
Why not “just bolus 500 mL”?
Trials/analyses across sepsis & critical illness link greater positive fluid balance to higher mortality & AKI. Repeating “trial boluses” often accumulates harm. Prefer reversible tests (PLR) and use congestion-aware strategies.
Physical Exam Tools (Pros & Cons)
JVP & Hepatojugular Reflux (HJR): rapid right-sided pressure clues; feasible and useful but operator dependent and harder in obesity/ventilation.
Peripheral edema / rales: late & nonspecific—note trend rather than absolute.
Pulse pressure (narrow) by palpation: suggestive of low SV, but confounded.
CRT/mottling: integrate with overall perfusion picture (temp, vasopressors).
Technology-Based Tools (non-POCUS)
Arterial line analytics
PPV/SVV (dynamic indices) are strong predictors of fluid responsiveness only when certain conditions are met: controlled ventilation, regular rhythm, VT generally ≥8 mL/kg (or use special maneuvers at lower VT), no spontaneous efforts, and no high intra-abdominal pressure.
Central Venous Pressure (CVP)
Useful for trends and certain etiologies (tamponade/obstruction) but does not predict fluid responsiveness.
Esophageal Doppler (ODM)
Provides beat-to-beat SV/CO; good for directed mini-fluid challenges or PLR; semi-invasive; operator dependent.
Pulse-contour CO devices (e.g., FloTrac)
Handy for trending, but accuracy can degrade in vasoplegia/arrhythmias/rapid tone shifts; mixed data vs thermodilution. Use judiciously.
Bioreactance/Thoracic bioimpedance (e.g., NICOM)
Non-invasive CO trending; measures phase shift (bioreactance) rather than amplitude; performance varies by setting (edema, chest tubes, arrhythmia).
Pulmonary Artery Catheter (PAC) / Swan-Ganz
Rich hemodynamics (PA pressures, PAOP, SvO₂) and helpful in complex RV failure/pulm HTN/mixed shock, but no mortality benefit in broad ICU RCTs; use selectively.
Pro tip: Pair any of the above with PLR (“internal bolus”) and track SV/CO change with echo, ODM, arterial waveform, or bioreactance to decide responsiveness while avoiding real fluid.
POCUS: Answering “Hypovolemic or Congested?”
1) IVC / IJ Veins
Diameter & variation can reflect RAP but are context-sensitive and often unreliable alone for predicting responsiveness (ventilation mode, pressure, habitus). Use as one tile in a mosaic, not the whole picture.
2) Lung Ultrasound (LUS)
B-lines = increased extravascular lung water (cardiogenic or non-cardiogenic). LUS is sensitive for pulmonary edema and useful to track de-resuscitation.
3) Focused Echo
Qualitative LV/RV function;
LVOT VTI to quantify SV changes with PLR/mini-bolus;
Assess RV size/strain if suspect right-sided issues (PE, ARDS-RV coupling).
4) VExUS (Venous Excess Ultrasound Score)
What: Integrated congestion score combining IVC size plus Doppler of hepatic, portal, and intrarenal veins.
Why: Moves beyond IVC to organ-level venous congestion; higher VExUS grades associate with AKI (especially after cardiac surgery) and can guide fluid removal strategies.
How (core patterns)
Hepatic vein: triphasic → biphasic/reversed with severe congestion
Portal vein: continuous → pulsatile with congestion
Intrarenal vein: continuous → biphasic/monophasic with severe congestion
Evidence snapshot: Original development work & subsequent cohorts show specificity for AKI risk, ongoing trials are testing VExUS-guided de-resuscitation.
-
ANDROMEDA-SHOCK
-
-