#64 JC4: End of 2025 Journal Club
This week we drop a journal club extravaganza! Should we do away with the A-Line? Is Bicarb a sure-fire way to treat metabolic acidosis in the setting of AKI? What about selective decontamination and all that capillary refill business!? We tackle all these topics through thoughtful journal discussion, provide our $0.02 and a little witty repartee, of course!Check it out and let us know what you think!
BICAR-ICU-2
Findings:
627 critically ill pts with pH ≤7.20 + KDIGO 2–3 AKI. Bicarb titrated to pH ≥7.30 vs usual care.
No difference in 90-day mortality: 62.1% vs 61.7% (absolute +0.4%, 95% CI −7.2 to +8.0; HR 0.97 [0.80–1.19]).A robust reduction in KRT use: 35% vs 50% (absolute −15.5%, HR 0.59 [0.46–0.75]) and delayed time to dialysis (31 h vs 16 h). MAKE90 similar.
Fewer bloodstream infections with bicarbonate (4% vs 9%). No major safety signal despite slightly increased early fluid load.
Context:
BICAR-ICU (2018) showed no overall benefit but a possible signal in the AKI subgroup. BICARICU-2 prospectively tested that subgroup and confirmed less KRT, but no survival benefit. Aligns with modern delayed-KRT strategies (AKIKI / IDEAL-ICU).
Criticisms:
Open-label → may influence clinician behavior, including when to start KRT.
Considerable crossover (~15–20% of controls received bicarb) → biases toward no mortality difference.
Powered for a very large 10% mortality reduction; observed rates likely made the study underpowered for smaller benefits.
Entirely French ICUs → KRT thresholds and practice patterns may differ internationally.
EVERDAC
Findings:
1,000 ICU pts with early shock (mostly septic; ~90% on vasopressors). Early art-line placement vs noninvasive BP monitoring.
28- and 90-day mortalities were very similar (34.3% vs 36.9%), meeting noninferiority criteria. Organ-support outcomes, ventilator-free days, and RRT use were likewise comparable.
Complications low but higher in invasive arm (8% hematoma/bleeding). Noninvasive group had much more arterial puncture burden (742 vs 269 attempts per 1,000 ICU-days) and reported more discomfort.
Context:
Art lines are nearly universal in shock management, but whether they improve outcomes has never been tested. This trial asked whether early routine catheterization is truly necessary for every patient.
Criticisms:
Excluded the sickest pts (high-dose NE, ECMO, severe TBI), the very group most likely to benefit from an art line.
~25% of the noninvasive group ultimately received an art line → high cross over rate implies that clinicians thought arterial lines were beneficial in a large fraction of the control group.
Wildly underpowered! Do you really think arterial lines would reduce mortality by 5%?!? This seems like an unreasonable expectation and thus a trial design that was doomed to fail. A more reasonable estimate of 1% would have required a larger trial or a different outcome.
Unblinded; decisions about escalation, gas sampling, and line placement may have differed by group.
Does not answer whether art lines improve outcomes in severe or refractory shock, only that routine early placement is not required for all —> we hopefully knew this already!
How does this change my practice: Not at all!
This study demonstrates that it is safe to defer immediate automatic placement of an arterial line in patients with shock. Hopefully we were all already already doing this!
For patients with persistent and severe shock, however, I usually proceed with catheterization, given the discomfort of noninvasive monitoring and serial arterial punctures, and the added safety of continuous blood pressure monitoring.
Arterial lines also permit less blood to be drawn for lab draws, potentially reducing transfusions. (an under-rated and in this study unmeasured potential benefit).
ANDROMEDA-SHOCK-2
Findings:
1,467 pts with early septic shock (lactate ≥2, on NE after ≥1 L fluids). CRT-guided algorithm vs usual care.
Primary outcome (hierarchical composite at 28 days using win ratio) favored CRT-guided therapy: win ratio 1.16 (95% CI 1.02–1.33; p=0.04).
Benefit came largely from more vital-support-free days (16.5 vs 15.4; proportional OR 1.28 [1.06–1.54]).
CRT normalization at 6 h higher (85.9% vs 61.7%) and achieved with less fluid (~600 vs ~850 mL in 6 h).
Dobutamine use higher (12.3% vs 5.3%).
Mortality unchanged (26.5% vs 26.6%; HR 0.99 [0.81–1.21]).
Hospital LOS similar.
Why this matters:
PROCESS/ARISE/PROMISE ruled out ScvO₂ and CVP-guided therapy; ANDROMEDA-1 suggested lactate clearance may be inferior to CRT. ANDROMEDA-2 tests a personalized, physiology-based algorithm, answering whether CRT-guided resuscitation improves meaningful clinical outcomes beyond mortality.
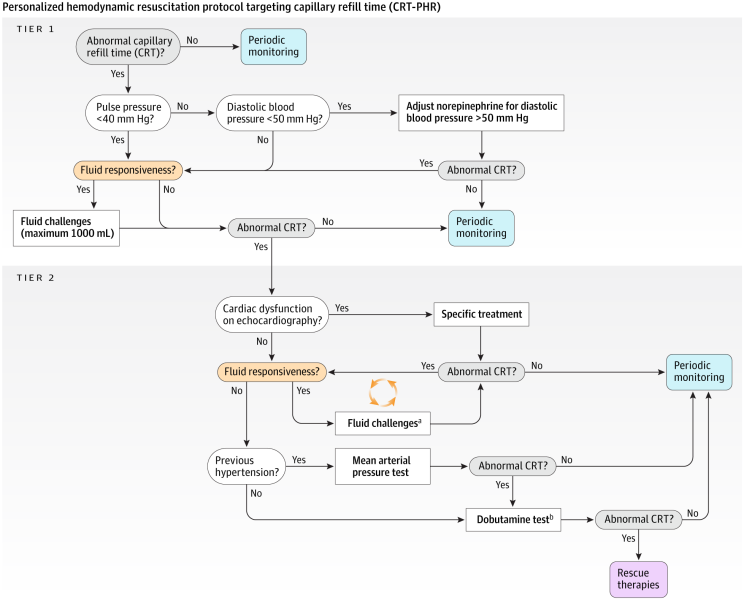
What was their physiological strategy:
Step 0 — Assess CRT
Press fingertip with a slide for 10 sec → measure time to color return.
If CRT normal → no escalation; reassess hourly.
If CRT abnormal (>3 sec) → proceed to Tier 1.
Tier 1 — Evaluate for Fluid Responsiveness & Vasodilation
Determine hemodynamic phenotype using:
Pulse pressure variation (PPV)
Diastolic arterial pressure (DAP)
Other dynamic tests allowed (PLR, SVV, EEOT/VTI)
If fluid responsive → give small fluid bolus (≈ 250–500 mL).
If vasodilatory physiology (DAP < 50 mmHg) → titrate norepinephrine to raise DAP and maintain MAP ≥65.
Reassess CRT.
If normalized → stop.
If still abnormal → Tier 2.
Tier 2 — Evaluate Cardiac Function & Escalate Therapy
Basic echocardiography to assess:
Ventricular function
Stroke volume response to fluids
Need for inotrope
Possible targeted interventions:
Repeat fluid responsiveness testing if appropriate
MAP “test”: raise MAP to 80–85 mmHg for 1 hr
Fixed-dose dobutamine (5 µg/kg/min) for 1 hr
Reassess CRT hourly for 6 hours total.
Key Principles
Stop giving fluids once CRT normalizes.
Individualize interventions based on physiology, not lactate alone.
CRT is the primary target; other tests guide how to correct it.
Strengths:
Large, multinational, highly pragmatic design → excellent external validity.
Physiologically grounded algorithm with stepwise escalation (fluids → DAP/MAP manipulation → dobutamine).
Use of win ratio appropriately prioritizes death first while also capturing differences in organ-support burden. (Nick is a big fan of win ratio in ICU clinical trials)
Demonstrated real behavioral impact: less fluid, more targeted hemodynamics, faster perfusion improvement.
High protocol adherence and reproducible physiologic assessments.
Supports bedside-focused, individualized resuscitation rather than reflexive fluid loading.
Criticisms:
Unblinded; decisions about extubation, RRT, vasopressor weaning may be biased.
Wide variability in tools for assessing fluid responsiveness (PPV, SVV, PLR, echo, IVC, EEOT) → operator-dependent, not standardized. Which is best? And for which operator/institution? Hard to operationalize.
Relatively complex algorithm may make implementation more difficult.
Uncertain accuracy of CRT assessment in darker skin tones.
Composite benefit driven by organ-support time rather than mortality → skeptics may question clinical significance.
What do we take away:
Nick’s perspective:
This is a win for personalized hemodynamic assessment! Go to the bedside & assess your patient!
DO try vasopressors early! (esp if low diastolic BP)
DO try dobutamine!
DO NOT just reflexively give fluids!
This is also a win for win-ratio and for well designed trials.
If they had used mortality alone as an endpoint we would talking about “another negative trail” instead of a game-changer
This is also a win for low tech: Low tech assessment coupled to a physiology based algorithm.
The challenge will be implementing CRT based resuscitation!
SuDDICU
Findings:
A large cluster-crossover RCT (~6,000 pts in Australia; ~9,289 with Canada). No significant mortality benefit at 90 days (Australia: 27.0% SDD vs 29.1% control; OR 0.91, 95% CI 0.82–1.02; combined data OR ~0.93, 95% CI 0.84–1.05). Ventilator-free days and LOS were unchanged.
However, SDD reduced ICU-acquired bloodstream infections (5.6% vs 8.1%) and reduced new antibiotic-resistant organism acquisition at the patient level (Australia: 23.1% vs 34.6%; combined: 16.8% vs 26.8%; adjusted difference ≈ –9.6%). C. diff rates low and similar.
Ecologic surveillance failed to meet non-inferiority for resistance, meaning SDD might increase unit-level resistance even though individual patients had fewer resistant isolates.
Why it mattered historically:
Prior meta-analyses (~24,000 pts) suggested reduced VAP, bacteremia, and a small survival benefit (RR ≈0.91). Benefits were mainly in low-resistance settings and often required an IV antibiotic component. SuDDICU was the first large RCT powered for mortality and designed to rigorously evaluate resistance ecology, the major barrier to adoption.
Criticisms:
Conducted largely in low-resistance regions (Australia/Canada) → unclear global generalizability.
~50% of controls received early broad-spectrum antibiotics mimicking the IV component of SDD → reduced between-group separation.
>1,200 protocol deviations in SDD arm → further dilution.
Ecologic analysis ambiguous; could not exclude a small increase in unit-wide resistance.
Did not directly measure VAP (central rationale for SDD).
Possibly underpowered for a small mortality effect consistent with prior meta-analyses.